19th december 2025
Funding Friday Series – Episode 4:
What "Breakthrough Innovation" Actually Means
With 65% of Step 1 applications rejected for insufficient proof of “breakthrough innovation,” the EIC Accelerator’s Excellence criterion is a rigorous, evidence-based standard. Success hinges on three pillars:
Evidence quality is key. Funded champions like Panntherapi and Mode Sensors succeed by presenting regulatory-grade proof and avoiding common red flags like unsupported claims or regulatory naivety. Evaluators focus on credible, independently validated data, not aspirations.
At Strategic Healthcare Advisors, we help innovators turn technical ambition into concrete, fundable proof.
In this episode, Dr Caroline Chauché reveals how to demonstrate breakthrough status and navigate the main Step 1 pitfalls.
👉 Deeper dive: See how Panntherapi and Mode Sensors quantified their breakthrough advantage — comparison table available on demand [contact@strategic-healthcare.eu].
12th december 2025
Funding Friday Series – Episode 3:
EIC Accelerator Success Pattern
With only 6–8% of applicants funded, the EIC Accelerator rewards strategic preparation, not chance. Success consistently comes down to three elements: complete teams that combine technical depth with regulatory and commercial expertise; credible market pull demonstrated through LOIs, pilots or strategic partnerships; and a clear European trajectory showing how value will be created in the EU over the next 24–36 months.
Sector dynamics also matter: medical devices account for more than a third of funded projects, while biotherapeutics, digital health and livestock technologies require strong differentiation to rise above the competition. The strongest applicants invest months in strengthening teams, generating evidence and shaping a compelling European growth story before applying.
At Strategic Healthcare Advisors, we support innovators in building these foundations and aligning their strategy with evaluator expectations.
In this episode, Dr Caroline Chauché outlines the real drivers of EIC Accelerator success — and what startups must do to position themselves effectively.
5th December 2025
Funding Friday Series – Episode 2:
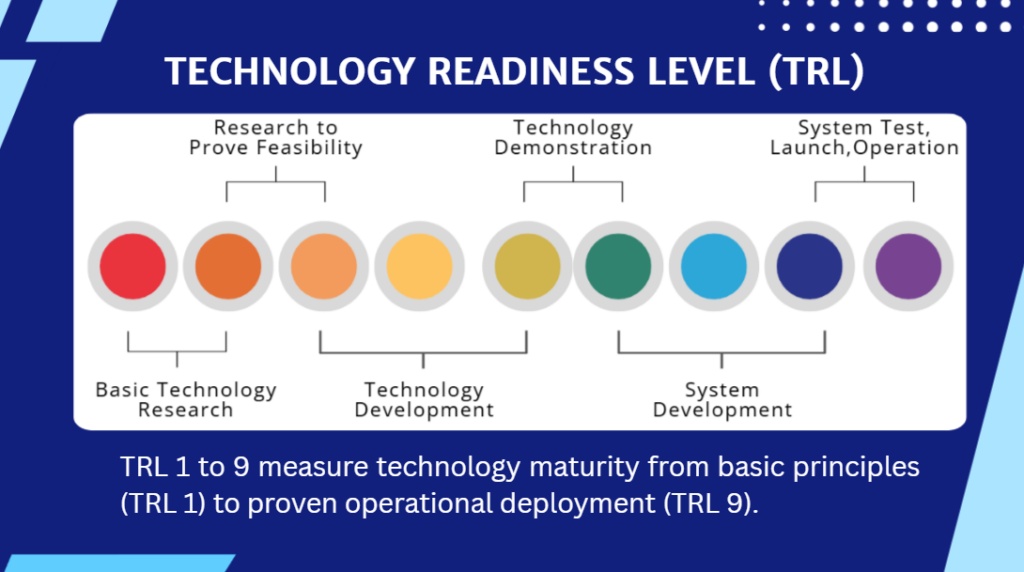
Understanding the TRL 6 Threshold Across Life Sciences
Reaching TRL 6 is now one of the most decisive factors in EIC Accelerator success — yet it remains one of the least understood. While the Work Programme requires applicants to enter at TRL 5, evaluators ultimately select companies that demonstrate a credible, evidence-driven trajectory toward TRL 6–8. Crucially, this threshold is not uniform: what constitutes “validated in a relevant environment” varies significantly across MedTech, Biotech Therapeutics, Digital Health, Deep Tech and Sustainable Livestock.
These sector-specific validation requirements—ranging from clinical-environment prototype testing to robust preclinical packages, prospective real-world data, dual platform–application validation, or complex field trials—explain why many promising innovations fall short. The differentiator is not scientific merit, but whether the evidence package reflects the standards regulators and evaluators expect for that technology category.
At Strategic Healthcare Advisors, we help innovators design validation strategies that align scientific, regulatory and funding pathways, ensuring TRL progression is both sector-appropriate and compelling to evaluators.
In this episode, Dr Caroline Chauché outlines what TRL 6 really means across life sciences sectors — and why understanding these differences is essential for EIC Accelerator readiness.
28th November 2025
Funding Friday Series - Episode 1:
The EIC Acceleration Work Programme 2026 - A Paradigm Shift
The 2026 EIC Accelerator introduces major changes that raise the bar for life sciences innovators, including €414 million for open calls, a doubled minimum equity investment of €1 million, and a mandatory TRL 5 threshold. These shifts prioritise later-stage companies with proven commercial traction and committed co-investors. While competition will become tougher, the programme remains Europe’s most powerful source of non-dilutive funding, offering recipients both substantial financial support and the prestigious EIC endorsement—a catalyst for 2–3x follow-on investment and accelerated partnerships or exits within 2–3 years.
Our team understands exactly what EIC evaluators and investors seek. Contact us to discuss your EIC Accelerator strategy.
In this episode, Dr Caroline Chauché explains what’s changed in 2026 and why it matters for life sciences innovations"
30th SEPTEMBER 2025
French HealthTech Startup in the United States in 2025
On September 30th, founders, investors, and industry experts came together to explore a key question: How can French HealthTech startups successfully expand to the United States? The discussion led to the creation of a new guide, “French HealthTech Startups in the USA – Your Guide to Break Through and Fund Success,” now available for download. With the U.S. capturing 45% of the global HealthTech market — and average funding rounds reaching $50–100M compared to just €2–3M in France — the stakes are high. The guide highlights practical success factors: adapting culturally, building teams with senior U.S. talent, structuring transatlantic operations, blending European and American funding, and balancing humility with agility.



4th August 2025
Don't Pitch Too Soon -Ask yourself these 8
Questions
Jean-Michel Verjus, Co-Managing Partner at Strategic Healthcare Advisors, has published an essay highlighting the key questions every healthcare startup should ask before raising funds. From defining the right timing and funding needs to choosing suitable investors and preparing a fallback plan, his guide helps founders ask themselves the right questions to avoid common mistakes and build a strong funding strategy.
At Strategic Healthcare Advisors, we support startups in tackling these challenges with confidence. By strengthening strategy and sharpening investor pitches, we help founders secure funding while staying in control of their company’s future.
1st-4th July 2025, Paris
Strategic Healthcare Advisors Supports Next-Gen Consultants
We’re proud to share that Francis Mathe, our Co-Managing Partner at Strategic Healthcare Advisors, recently served as a coach at SKEMA Business School’s 12th Digital Consulting Week. This event brings together students and industry leaders to solve real-world business challenges, giving participants valuable hands-on experience and exposure to future career paths.
Francis’s contribution reflects our core values of insight, collaboration, and impact. By supporting the next generation of digital and healthcare consultants, we continue to nurture talent and strengthen the future of our industry.


26th June 2025, Online
Cracking the code - Online Roundtable - Case study Epipole
Caroline Chauché, our Co-Managing Partner at Strategic Healthcare Advisors, recently hosted a roundtable with Scottish Development International to support epipole, a Scottish MedTech startup scaling into the French healthcare market. The session, titled “Cracking the Code: Entering the French Healthcare Market”, brought together leading experts in procurement, legal, IP, distribution, sustainability, and investment.
Key insights highlighted the importance of strong local partnerships, early legal and regulatory preparation, and the growing role of AI and sustainability in French hospital procurement. This collaborative effort reflects our commitment to guiding innovators like epipole through complex markets with clarity and impact.
Entering the French Healthcare Market - Cracking the Code Online roundtable Summary and TakeawaysDownload

19th - 21st May 2025, Paris
Strategic Healthcare Advisors Supports Scottish Innovation at SantExpo 2025
Strategic Healthcare Advisors (SHA) supported Scottish Development International (SDI) and accompanied a delegation of Scottish healthcare start-ups to SantExpo 2025, a leading European healthcare innovation event.
The SHA team provided end-to-end support, from organising a pre-exhibition networking dinner to on-site guidance at the SDI stand, helping start-ups navigate meetings, cultural differences, and increase visibility through targeted media coverage. SHA also supported SDI in hosting a private reception at the British Embassy in Paris, facilitating high-value introductions with potential buyers.
A key highlight was a panel discussion hosted by SHA, bringing together European healthcare innovation experts to explore the future of medical innovation. This collaboration reinforces SHA’s role as a trusted partner in supporting international healthcare delegations and accelerating market access.